The diminishing conventional energy resources based on fossil fuels and their related environmental consequences have drawn attention around the world toward the development of renewable energy resources. These renewable energy resources may not fulfill the entire energy demands of world’s mass population; however, they limit the effects of greenhouse gases as well as air pollution caused by the fossil fuel burning. Among alternative resources, hydrogen is considered to be the cleanest energy carrier.
However, hydrogen does not exist in its pure state in nature, like oxygen, and has to be produced from hydrogen-containing resources such as natural gas (methane), coal, biomass and water by reforming, thermal decomposition or electrolysis. But production of hydrogen from natural gas, coal and biomass leads to the emission of the greenhouse gas carbon dioxide (CO2).
We know that water (H2O) is made of hydrogen and oxygen atoms; hence, sea water could be a limitless source of hydrogen. Therefore, hydrogen is envisaged as a possible replacement for fossil fuels. Production via power from renewable energy (using wind power, solar power, hydropower, wave power or similar) is termed as “green hydrogen.” In this scenario, splitting water into hydrogen and oxygen using renewable electricity in an electrolyzer on the surface of a robust electrocatalyst is one proposed technique.
Need for a robust electrocatalyst
Despite advancements in the field, the process of water spitting to produce affordable green hydrogen still remains sluggish due to limitations related to efficient electrocatalysts. In theory, water splits at 1.23 V. However, in practical terms, this value is greater than 1.5 V (meaning wastage of additional energy). This minimum energy is theoretically required to break the water molecule. Expensive noble- and precious-metal-based electocatalysts, for instance, Pt, Pd, Au, Rh, Ir, etc., are used in the electrolyzer for this process.
The main problems the industry and experts face are the oxidation of water to produce O2 and the stability of the catalyst in harsh industrial alkaline conditions. In the first problem, the half-cell reaction is an uphill reaction where four electrons are involved and where most of the energy is required apart from the energy loss associated with the resistivity of different components (electrolyte, connections, catalyst, etc.) of the electrolyzer. In the second problem, the expensive catalysts often lose their activity due to surface degradation. In these conditions, a cheap and affordable yet highly active and stable electrocatalyst is required for such water splitting reaction.
Present development
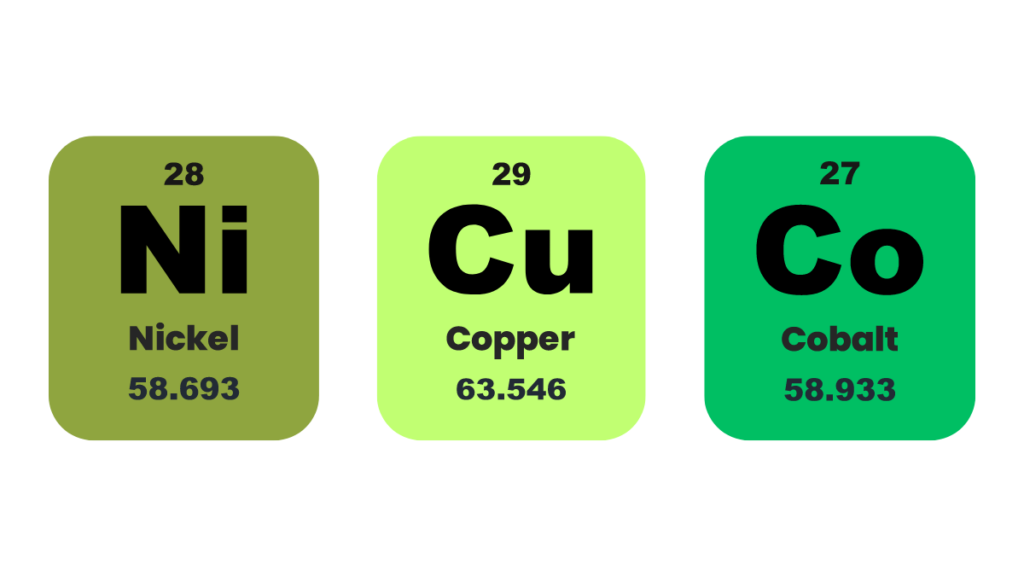
In a recent study, our team, led by Sasanka Deka, designed and developed a new nanocomposite-based, highly efficient, yet still cost-effective electrocatalyst for overall water splitting. A nanocomposite is a homogeneous mixture of two or more materials present in the nanometer range. The present nanocomposite is a nanoarchitecture based on NiCu dealloyed nanoparticles on hierarchical Co nanosheets. Our findings are published in the journal ACS Catalysis.
The materials used are cheaper than the precious metals and the synthesis procedure is highly convenient. This new catalyst was used…